Wasted implants contribute to high costs of spinal surgery
Can epidural stimulation help paralysed patients to stand and walk?



A study presented in March at the ISASS Annual Meeting, Las Vegas, USA, has shown that material waste during spinal surgery is considerably higher than that published in the trauma and arthroplasty literature, with estimated annual costs of US$127million every year in the USA. The study also showed that the implementation of an awareness programme reduced monthly costs attributable to intra-operative waste by 66% in one US institution
“Prior studies have demonstrated that surgical implant
waste is a factor influencing cost in arthroplasty and orthopaedic trauma.
However, the role of intra-operative waste has not yet been studied in the
context of spinal surgery,” Alexandra Soroceanu, Department of Orthopaedic
Surgery, Dalhousie University, Halifax, Canada, told delegates at the
ISASS (The International Society for the Advancement of Spine Surgery,
formerly the Spine Arthroplasty Society) Annual Meeting. “This study aimed
to quantify the incidence of intra- operative waste in spinal surgery, and
to examine the efficiency of an awareness programme directed at surgeons
in inducing a decrease in the intra-operative waste.” Each year, over
600,000 surgical interventions are performed on the spine in the United
States. Spine pro- cedures are known for their associated high cost,
particularly in cases where instrumentation is employed. Intra-operative
waste has been defined as products that were prepared but not used during
the surgery, and which cannot be re-used for a different patient. The
investigators collected data for 25 months (from October 2007 to November
2009) from one US academic centre. During the initial observation period
(15 months), a total of 1,304 spine procedures were performed. In that
time, intra-operative waste occurred in 263 cases (20.2%). This
corresponded to a total number of 739 items, amounting to a total of
US$275,356. Surgical implants accounted for only 42% of the number of
items wasted. However, surgical implants accounted for a cost burden of
US$234,868 or 85.3% of the cost of all waste during the initial
time-frame. Contamination and surgeon’s change of mind accounted for the
majority of the wasted items. After an initial observation period of 15
months, Soroceanu said, an awareness programme was put in place. “As part
of the programme, all spinal surgeons and operating room staff were made
aware of what constituted intra-operative waste. Surgeons were also
presented with the data of costs associated with surgical waste, both on
an institutional and an individual level monthly without anonymity. Data
were collected for an additional 10 months after the intervention,” she
noted. Ten months after the implementation of the programme, the results
showed that the incidence of intra-operative waste had fallen to 10.3%
(p<0.0001). Monthly costs associated with surgical waste were, on
average US$17,680.29 prior to the awareness intervention, and US$5,876.87
afterwards (p=0.0006). “Our awareness programme allowed us to decrease the
number of implants wasted (OR 0.434, p<0.001), and the incidence of
surgeons’ change of mind (OR 0.41, p<0.001),” Soroceanu said.
“According to our results, the incidence of surgical waste related to
spinal surgery is considerably higher than that published in the trauma
and arthroplasty literature. Prior to the educational intervention, the
annualised cost related to surgical waste was US$212,000 at our
institution. Extrapolation of our data to the national level leads to an
annual estimate of US$127million attributable to intra-operative waste,”
she told delegates. Soroceanu said, “A programme that made surgeons -aware
of costs related to surgical waste allowed us to reduce waste costs by
66%. This study demonstrates that surgical waste is an important cost in
spine surgery, and that surgeon awareness may help contribute to decrease
the cost burden.”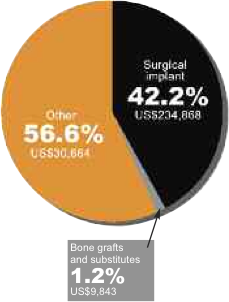
A case study published in The Lancet in May gathered tremen- dous media attention around the world. It showed that epidural stimulation enabled a 23-year-old man who had para- plegia from a C7–T1 subluxation to stand (with assistance provided only for balance) for 4·25min. The researchers also showed that seven months after surgical implanta- tion of a 16-electrode array on the dura (L1–S1 cord segments) in December 2009, the patient recovered supraspinal control of some leg movements, but only during epidural stimulation. The team of researchers from the University of California, Los Angeles (UCLA), the California Institute of Technology (Caltech), and the University of Louisville have shown that using a stim- ulating electrode array has assisted a paral- ysed man to stand, step on a treadmill with assistance, and, over time, to regain volun- Continued on page 4 tary movements of his limbs. They have found that the electrical sig- nals provided by the array stimulate the spinal cord’s own neural network so that it can use the sensory input derived from the legs to direct muscle and joint movements. The patient, Rob Summers, had the sub- luxation as a result of a motor vehicle acci- dent and presented with complete loss of clinically detectable voluntary motor func- tion and partial preservation of sensation below the T1 cord segment. Leading researchers on the 11-member team are two prominent neuroscientists: Susan Harkema, of the University of Louisville’s Department of Neurosurgery, Kentucky Spinal Cord Research Center and Frazier Rehab Institute, a service of Jewish Hospital & St. Mary’s HealthCare in Louisville; and V Reggie Edgerton, the Division of Life Sciences and David Geffen School of Medicine at UCLA. Joel W Burdick, professor of Mechanical Engineering and Bioengineering at Caltech, developed new electromechanical technologies and computer algorithms to aid in locomotion recovery in spinal cord injury patients. The researchers wrote in The Lancet that they hypothesised that tonic epidural spinal cord stimulation could modulate spinal circuitry in human beings into a physiological state which enables sensory input from standing and stepping move- ments to serve as a source of neural con- trol to undertake these tasks. After 170 locomotor training sessions over 26 months, a 16-electrode array was surgically placed on the dura (L1–S1 cord segments) in December 2009, to allow for chronic electrical stimulation. Spinal cord stimulation was done during sessions that lasted up to 250min. “We did 29 experi- ments and tested several stimulation com- binations and parameters with the aim of the patient achieving standing and step- ping,” wrote the researchers. A statement from the Christopher and Dana Reeve Foundation, which funded the study along with the National Institutes of Health said “These unprece- dented results were achieved through con- tinual direct epidural electrical stimula- tion of the subject’s lower spinal cord, mimicking signals the brain normally transmits to initiate movement. Once that signal is given, the research shows, the spinal cord’s own neural network com- bined with the sensory input derived from the legs to the spinal cord is able to direct the muscle and joint movements required Susan Harkema to stand and step with assistance on a treadmill. The other crucial component of the research was an extensive regime of Locomotor Training while the spinal cord was being stimulated and the subject sus- pended over the treadmill. Assisted by rehabilitation specialists, the individual’s spinal cord neural networks were retrained to produce the muscle move- ments necessary to stand and to take assisted steps. “The spinal cord is smart,” noted Edgerton, distinguished professor of inte- grative biology and physiology, and neu- robiology at UCLA. “The neural net- works in the lumbosacral spinal cord are capable of initiating full weight-bearing and relatively coordinated stepping with- out any input from the brain. This is pos- sible, in part, due to information that is sent back from the legs directly to the spinal cord.” This sensory feedback from the feet and legs to the spinal cord facili- tates the individual’s potential to balance and step over a range of speeds, directions and level of weight bearing. The spinal cord can independently interpret these data and send movement instructions back to the legs, all without cortical involve- ment. “This is a breakthrough. It opens up a huge opportunity to improve the daily functioning of these individuals,” con- cludes Harkema, lead author of The Lancet paper. “But we have a long road ahead.” “While these results are obviously encouraging,” concurs Edgerton, “we need to be cautious. There is much work to be done.” To begin with, only one subject has been studied and he was an athlete in extraordinary physical condition before his injury. (Five human subjects have been authorised by the Food and Drug Administration to be enrolled in the study.) Additionally, the first subject, while completely paralysed below the chest (C7/T1 vertebra spinal section), was rated “B” on the American Spinal Injury Association’s classification system, since he did retain some feeling below the level of injury. It is not known how these inter- Rob Summers ventions will work with “A”-level patients (no cognition of sensation below the injury). Yet another issue is the stimulation equipment itself. To date, researchers have only had access to standard off-the-shelf stimulation units designed for pain relief. Also, in earlier published animal stud- ies, drug interventions further heightened the sensitivity and functioning of the spinal cord’s neural network. The com- pounds used in animals, however, are not approved for human use. Harkema et al conclude in The Lancet article that “Task-specific training with epidural stimulation might re-activate previously silent spared neural circuits or promote plasticity. These interventions could be a viable clinical approach for functional recovery after severe paralysis.”